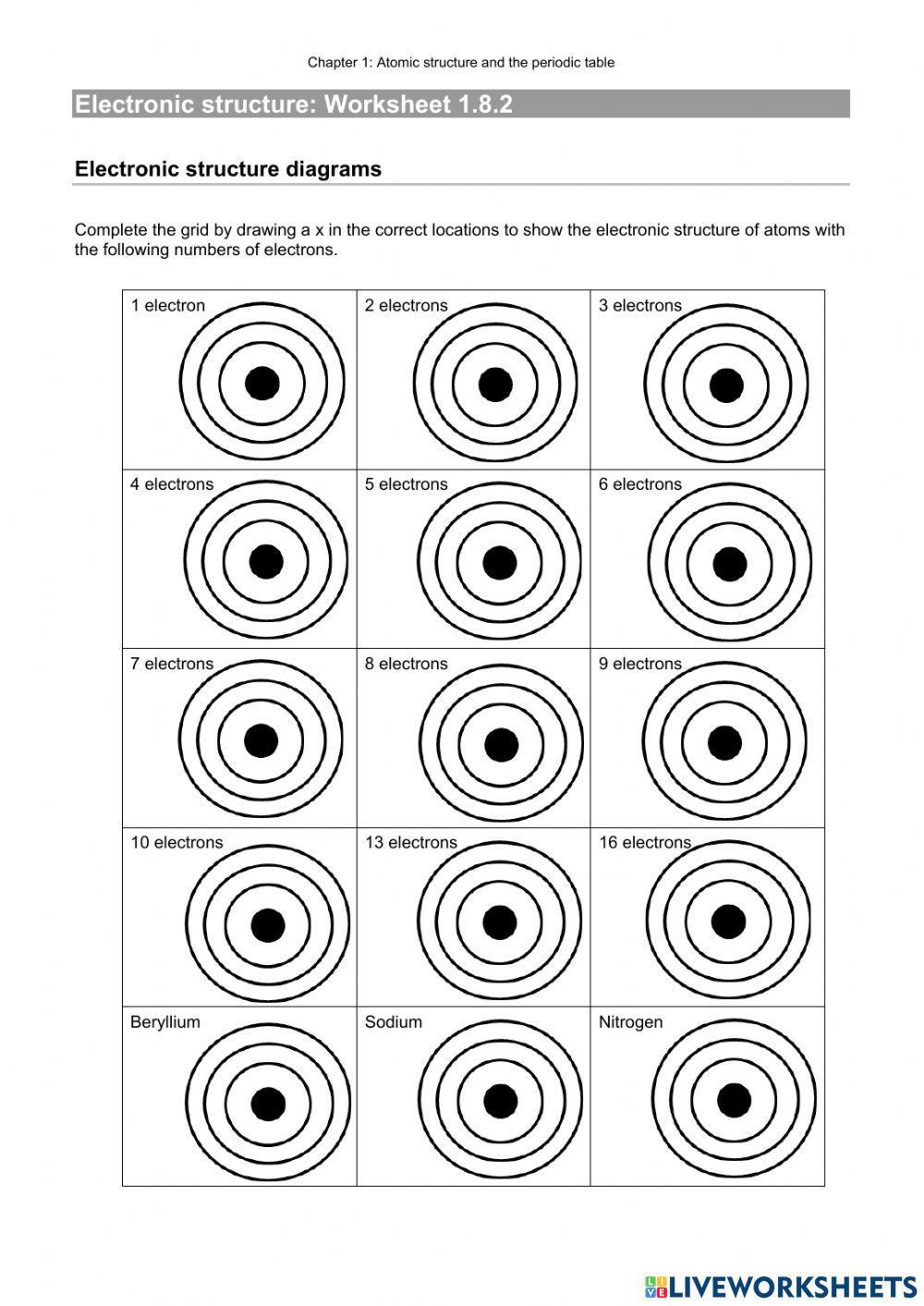
Electron Configuration Worksheet
This worksheet from Teach Simple tests students knowledge of standard and noble gas electron configuration how to read configurations and find mistakes in them An answer scheme is provided to allow for peer marking Suitable for Grade 8 upward Download Here Brief Instructions An electron configuration is a method of indicating the arrangement of electrons about a nucleus. A typical electron configuration consists of numbers, letters, and superscripts with the following format: A number indicates the energy level (The number is called the principal quantum number.).

Electron Configuration Worksheet This worksheet provides extra practice for writing electron configurations The easiest and most reliable technique for writing electron configurations is to use the periodic table as your guide Details of Electron Configurations - Solutions. Note: The electron configurations in this worksheet assume that lanthanum (La) is the first element in the 4f block and that actinium (Ac) is the first element in the 5f block. If your periodic table doesn’t agree with this, your answers for elements near the f-orbitals may be slightly different. 1) sodium
Electron Configuration Worksheet
This infographic is designed to be displayed as a poster in the classroom although it can also be displayed on a projector or printed as a handout Use the accompanying fact sheet and worksheet to get your students drawing electron configuration diagrams Electron configuration worksheet answers key pro worksheet. Free printable electron configuration orbital diagram worksheetsElectronic configuration worksheets.

13 Best Images Of Electron Configuration Worksheet With Answers

Orbital Diagram And Electron Configuration Worksheet Prntbl
Electron configurations Google Classroom You might need Periodic table Using spdf spdf notation what is the electron configuration for a neutral atom of beryllium Give the electron configurations of atoms and ions using subshells and orbitals including Hund’s rules. Use different representations of electron configurations including noble gas cores and ‘electrons in boxes’. Relate the subshell containing the outermost electrons to an element’s position in the periodic table.
The following electron configurations belong to which elements 21 1s22s22p63s1 sodium 22 1s22s22p63s23p64s23d104p65s24d6 ruthenium 23 Kr 5s24d10 cadmium 24 Xe 6s24f145d106p2 lead 25 Rn 7s25f146d4 seaborgium Determine if the following electron configurations are correct 26 1s22s22p63s23p64s24d104p65s1 no it should be 3d10 This worksheet provides extra practice for writing electron configurations. The easiest and most reliable technique for writing electron configurations is to use the periodic table as your guide. Details of using the periodic table as a guide for determining electron configurations can be found on the CH301 website.