Mean Free Path Formula
Formula of Mean Free Path Mathematically representation of the mean free path can take place as follows frac 1 sqrt 2 pi d 2 frac N V Derivation of the Formula of Mean Free Path The derivation of the equation will make use of certain assumptions Consider the molecule to be spherical The mean free path is the average path covered by the molecules between collisions. It is known that there are different free paths with different path lengths. Given below are the free paths, λ 1 = First free path. λ 2 = Second free path. λ 3 = Third free path. λ n = nth free path.
A gas has an average speed of 10 m s and an average time of 0 1 s between collisions What is its mean free path A gas has a density of 10 particles m 3 and a molecular diameter of 0 1 m What is its mean free path Answer l 1 pi d 2 N v 1 pi 0 1 m 2 10 m 3 2 25 m Assuming that all the target particles are at rest but only the beam particle is moving, that gives an expression for the mean free path: = (), where ℓ is the mean free path, n is the number of target particles per unit volume, and σ is.
Mean Free Path Formula
The free paths are listed below with 1 1 denoting the first free path and 2 2 The second free path 3 3 the third free path n n the nth free path The mean free path is the average of these path lengths As a result it can be calculated as 1 2 3 n n 1 2 3 n n Ppt chemistry 232 powerpoint presentation free download id 2214653. Diffusion why is the mean free path divided by sqrt 2 physics Estimate the mean free path and collision frequency cbse class 11 .

The Mean Free Path Lambda Of Molecules Is Given By Where N Is The

Translation Kinetic Energy And Mean Free Path YouTube
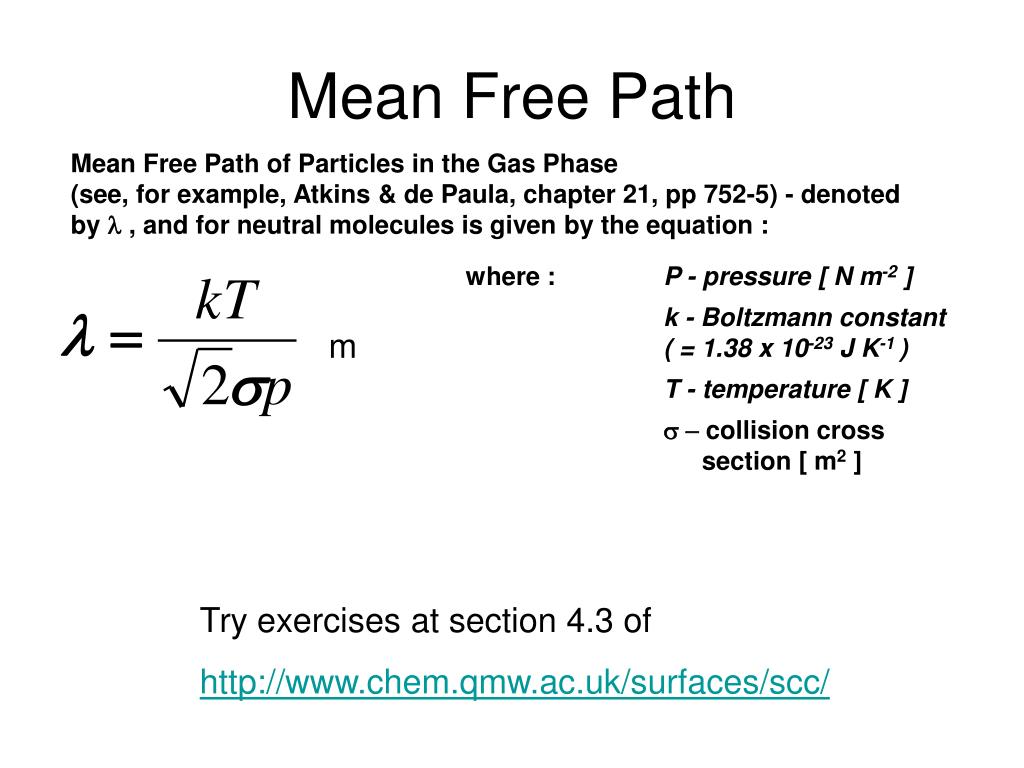
Our mean free path calculator uses the below mean free path equation lambda frac k rm B T sqrt 2 pi d 2p 2 d2pkBT where lambda Mean free path expressed in the length units T T T Temperature of the gas p The mean free path equation depends upon the temperature and pressure as well as the molecular diameter. For pressure P 0 = mmHg = inHg = kPa. and temperature T= K = C = F, Molecules of diameter x 10-10 meters (angstroms)
Collision theory is a theory proposed independently by Max Trautz in 1916 and William Lewis in 1918 that qualitatively explains how chemical reactions occur and why reaction rates differ for different reactions The mean free path is the distance a particle will travel, on average, before experiencing a collision event. This is defined as the product of the average speed of a particle and the time between collisions. The former is v , while the latter is 1 / zA. Hence, we have. λ = v √2ρσ v = 1 √2ρσ.