Structure Of Atom Notes
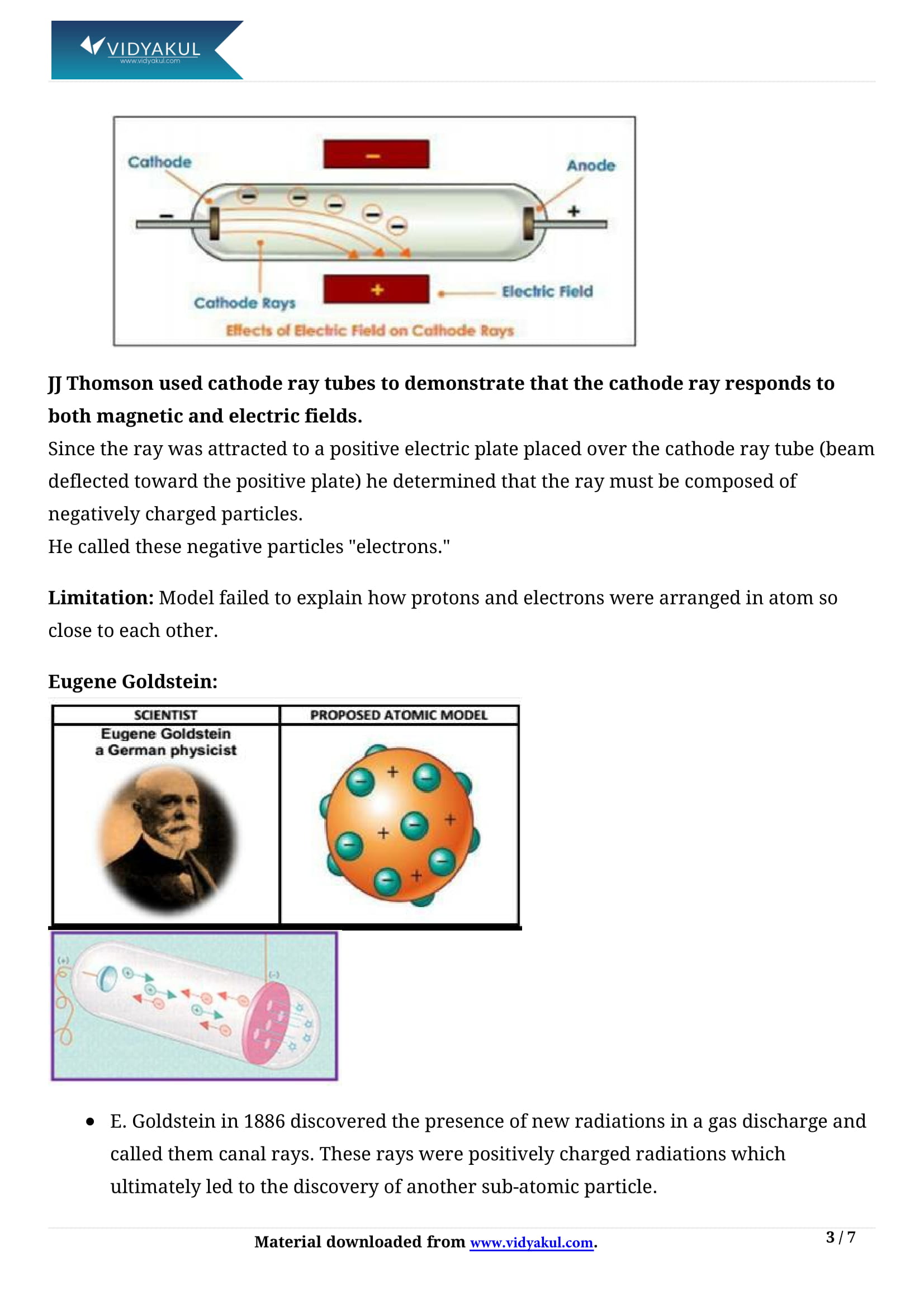
Figure 2 2 1 2 2 1 The Structure of the Atom Atoms have protons and neutrons in the center making the nucleus while the electrons orbit the nucleus The modern atomic theory states that atoms of one element are the same while atoms of different elements are different Introduction to the Structure of an Atom Atoms. Atoms are the building blocks of matter. It is the smallest unit of matter that is composed of three sub-atomic particles: the proton, the neutron and the electron. To know more about Atomic Structure, visit here. Cathode Ray Experiment. J. J. Thomson discovered the existence of electrons.

Home Study Guides Chemistry Structure of the Atom Structure of the Atom Atoms are the smallest units of elements A block of iron is made up of a huge number of iron atoms packed together Atoms are also composed of still smaller particles of matter protons neutrons and electrons The atomic structure refers to the structure of an atom comprising a nucleus (centre) in which the protons (positively charged) and neutrons (neutral) are present. The negatively charged particles called electrons revolve around the centre of the nucleus. Download Complete Chapter Notes of Structure of Atom Download Now

Structure Of Atom Notes
Atoms contain protons neutrons and electrons The electrons are arranged in shells around the nucleus Part of Chemistry Single Science Atomic structure and the periodic table A history of the atom theories and models guernseydonkey. Writing notes on my form 3 chemistry for revision on atomic structure Structure of atom class 9 notes edurev.
Structure Of The Atom Class 9 Notes Vidyakul

Write A Short Note On Rutherford s Model Of Atom With A Diagram Class
Most of the atom is empty space The rest consists of three basic types of subatomic particles protons neutrons and electrons The protons and neutrons form the atom s central nucleus The ordinary hydrogen atom is an exception it contains one proton but no neutrons As their names suggest protons have a positive electrical charge We now know a great deal about the structure of an atom. We know that the atom has a nuclear structure, we know that the positive charges and mass of the atom are concentrated in the nucleus, and we know how.
The structure of atom consists of two parts an atomic nucleus extra nucleus part The tiny atomic nucleus is the center of an atom It constitutes positively charged particles protons and uncharged particles neutrons On the other hand the extra nucleus part is a much larger region It consists of a cloud of negatively charged Physical Chemistry (Essentials) - Class 11 8 units · 52 skills. Unit 1 Welcome to physical chemistry. Unit 2 Structure of atom. Unit 3 Some basic Concepts of Chemistry. Unit 4 Redox reactions. Unit 5 Gaseous state. Unit 6 Thermodynamics. Unit 7 Chemical Equilibrium. Unit 8 Ionic equilibrium.