What Is The Rate Of Reaction
2 5 Reaction Rate Definition of Reaction Rate The Reaction Rate for a given chemical reaction is the measure of the change in Instantaneous rates Most reactions slow down as the reactants are consumed Consequently the rates given by the Rate Laws and Rate Constants A rate law is an reaction rate, in chemistry, the speed at which a chemical reaction proceeds. It is often expressed in terms of either the concentration (amount per unit volume) of a product that is formed in a unit of time or the concentration of a reactant that is consumed in a unit of time.

The rate of a reaction is a measure of how quickly a reactant is used up or a product is formed Collision theory For a chemical reaction to happen reactant particles must collide with each The reaction rate or rate of reaction is the speed at which a chemical reaction takes place, defined as proportional to the increase in the concentration of a product per unit time and to the decrease in the concentration of a reactant.

What Is The Rate Of Reaction
Rate Laws and Reaction Order The relation between the rate of a reaction and the concentrations of reactants is expressed by its rate law For example the rate of the gas phase decomposition of dinitrogen pentoxide 2N 2O 5 4NO 2 O 2 has been found to be directly proportional to the concentration of N 2O 5 text rate k N Ppt rate of reaction powerpoint presentation free download id 2483456. Factors that affect rate of reaction good sciencePpt rate of reaction powerpoint presentation free download id 2483456.
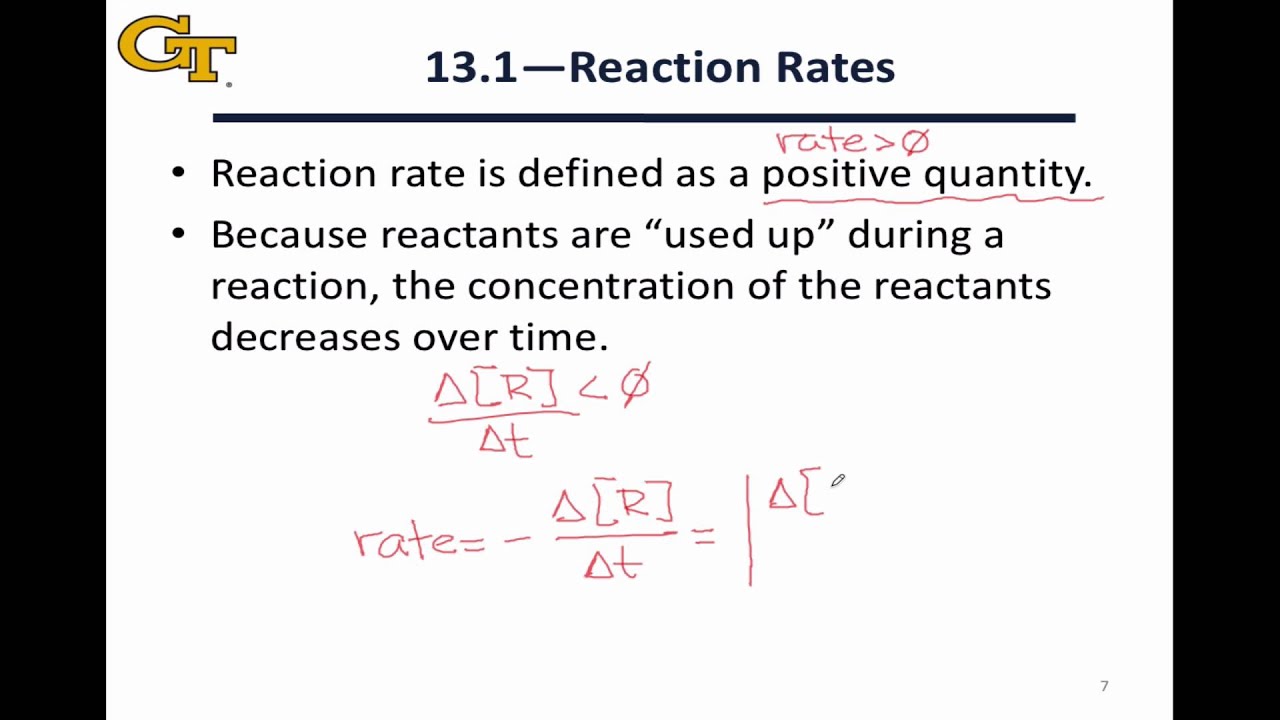
13 1 Definition Of Reaction Rate YouTube
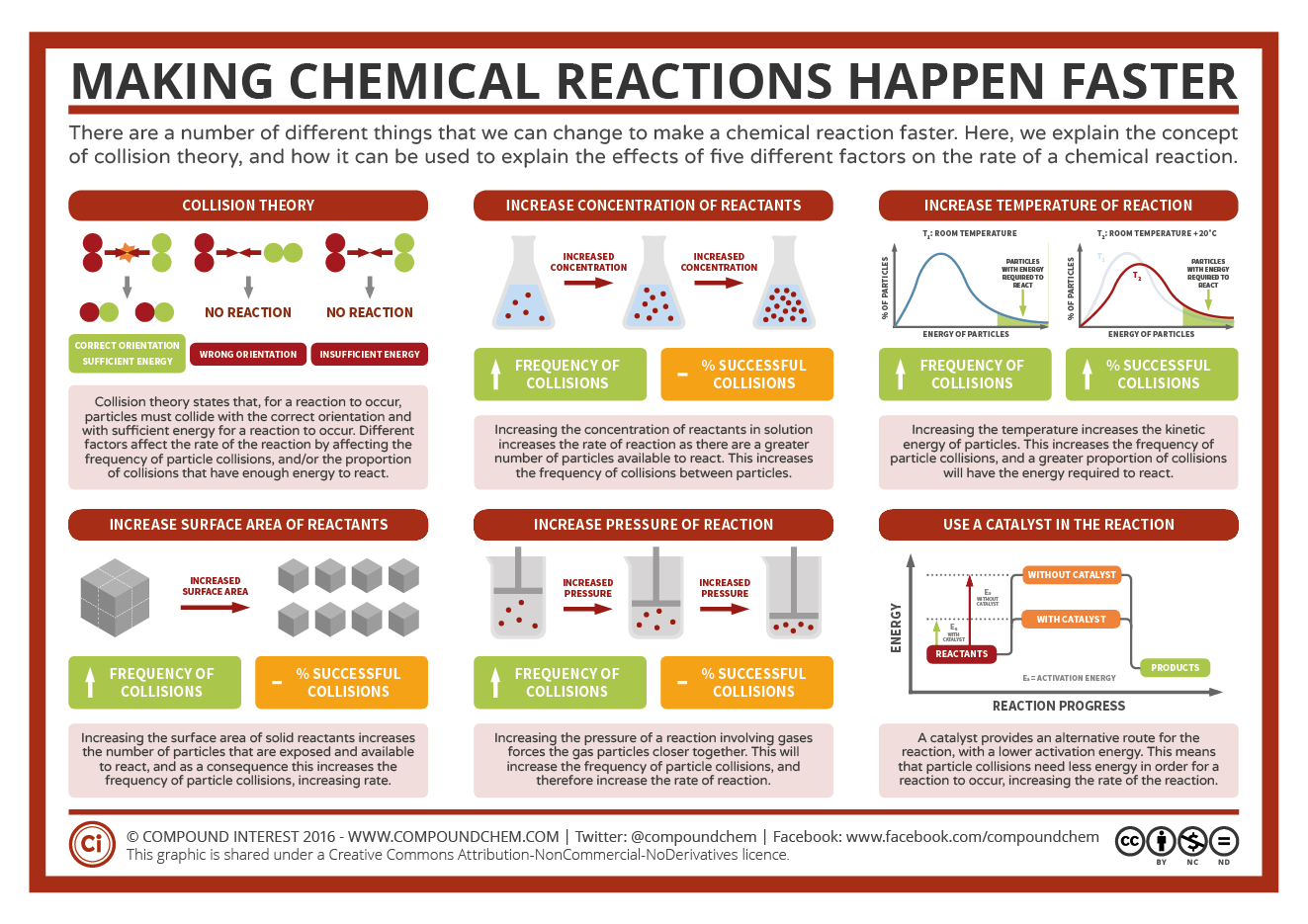
Making Reactions Faster Factors Affecting Rates Of Reaction Compound
Chemists have identified many factors that affect the rate of a reaction The rate or speed at which a reaction occurs depends on the frequency of successful collisions Remember a successful collision occurs when two reactants collide with enough energy and with the right orientation What is a rate of reaction? The rate of a reaction is a measure of how quickly a reactant is used up, or a product is formed. Collision theory For a chemical reaction to happen: reactant.
The reaction rate is defined as the rate at which the reactants of a chemical reaction form the products Reaction rates are expressed as concentration per unit time Reaction Rate Equation The rate of a chemical equation can be calculated using the rate equation For a chemical reaction a A b B p P q Q The rate of the reaction is The rate of a chemical reaction is defined as the rate of change in concentration of a reactant or product divided by its coefficient from the balanced equation. A negative sign is used with rates of change of reactants and a positive sign with those of products, ensuring that the reaction rate is always a positive quantity.